https://en.wikipedia.org/wiki/Hyperoxia
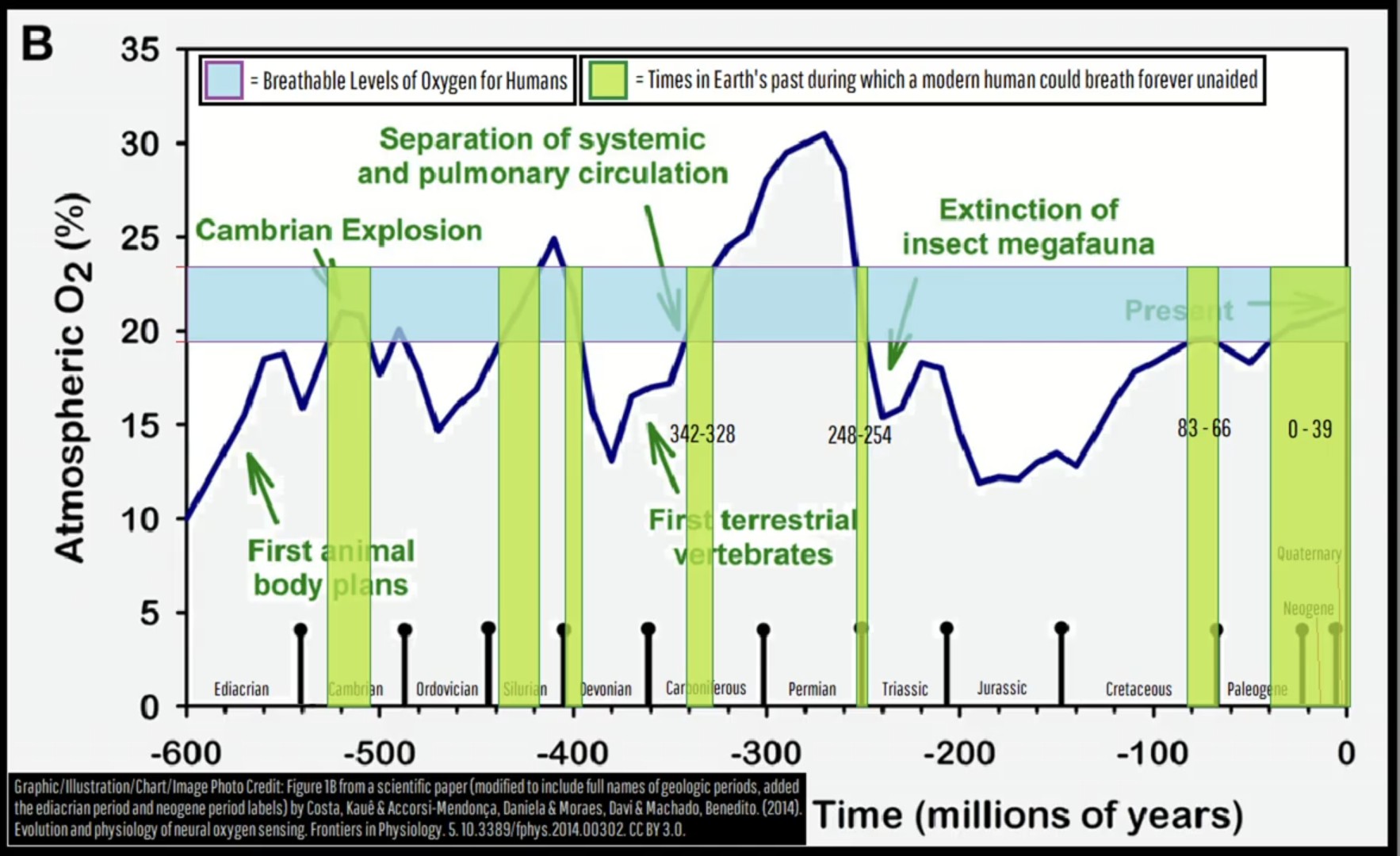
This was a screenshot I took months ago while watching a Geology Hub upload on YT. It was a lightbulb moment for my understanding of mass extinction events, (the largest was 250ma). I’ve referenced this multiple times, so thought I might share. Perhaps you find it as interesting as I do.


There are 850k people living in La Paz, Bolivia (elevation 3650m) with the equivalent of 13.2% sea level oxygen and they seem to be doing just fine. And granted, the natives of the region display some hemoglobin adaptation, but still… Even Aspen, Colorado sits at about 15% sea level oxygen and I’m pretty sure people don’t wear breathing gear while skiing there.
Altitude reduced the density of air as whole. The graphic is about changes in composition while density should remain similar, meaning if oxygen is low, something else (e.g. carbon mono-/dioxide) is high. So I wonder how this compares.
Partial pressure of the gases you breathe is what matters though, that’s why astronauts could breathe pure oxygen for days during the Mercury/Gemini/Apollo missions and be fine (as long as there’s no fire :/ ).
Just to clarify, the partial pressure of oxygen in the atmosphere is about 3 PSI. The partial pressure of Nitrogen in the atmosphere is about 10 PSI.
Fire risk is also related to the partial pressure of the gas. 100% oxygen is not actually a problem, so long as the partial pressure of that oxygen is low. The partial pressure of oxygen in the Apollo 1 capsule was about 16.7 PSI.
Astronauts still use 100% oxygen in space suits pressurized to about 5PSI.
I had always assumed they just compensated with oxygen pressure to match atmospheric partial oxygen pressure, but I checked and indeed you’re right, but it was only that high an oxgen pressure for testing purposes on the ground. Once in flight, they would have dropped it to 5 PSI. It also makes sense as to why that Apollo 1 fire was so violent. Thanks for that learning opportunity ! :)
I was actually more referring to the chart implying that low oxygen means higher concentration of toxic gases
Though you start to notice it around 2500m, as a untrained person.